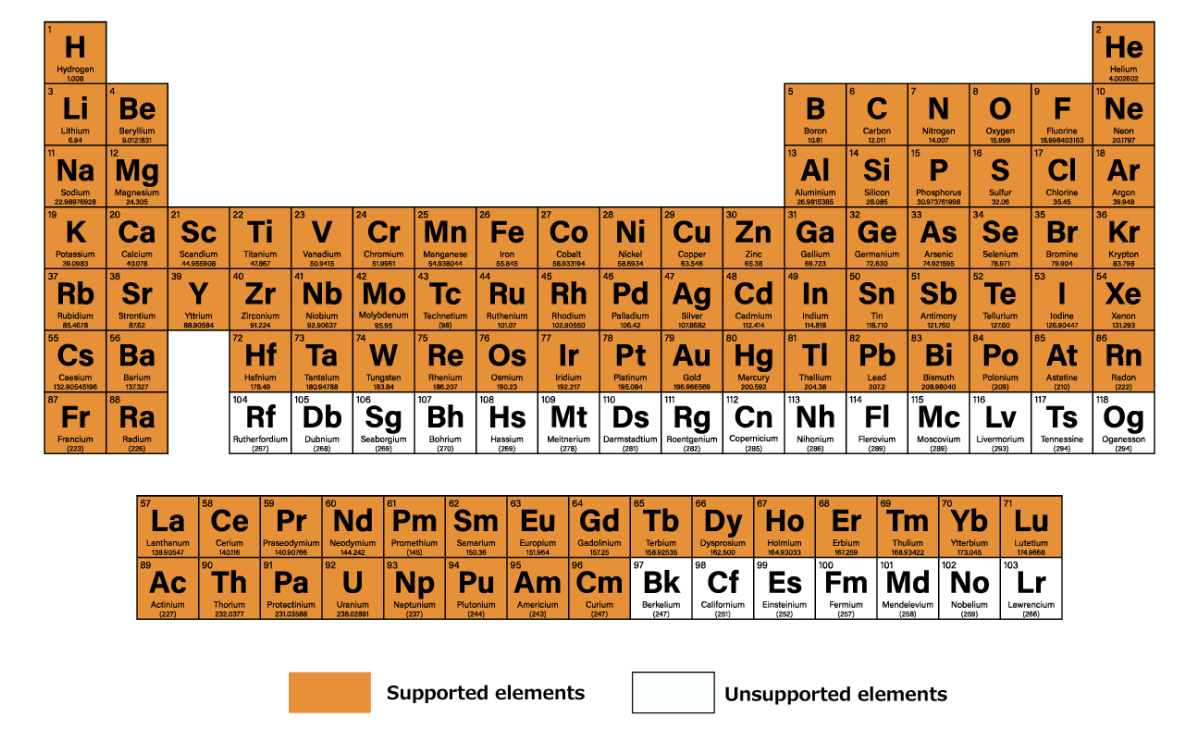
SiO₂ Dry Etching Simulation Using LightPFP
Case Study Provider:
Preferred Networks, Inc.

Overview
In semiconductor manufacturing, the etching of silicon dioxide (SiO2, also known as silica) layers is a core microfabrication technique. As integrated circuits become more advanced, dry etching has become essential for precise patterning and has been driving active research and development. Dry etching utilizes active species, such as plasma, to achieve high aspect ratios and selectivity, maintaining clean etched surfaces by removing reaction products as gases. Hydrogen fluoride (HF) , a highly reactive fluorine-based gas, is widely studied for dry etching because it generates volatile gases such as silicon tetrafluoride (SiF4), through reactions with SiO2[1]. However, experimentally observing the etching process at the nanoscale is difficult, and our atomic-level understanding remains limited.
This study developed a LightPFP model to simulate SiO2 dry etching with HF gas, leveraging active learning techniques. Our reaction pathway analysis with LightPFP showed DFT-level accuracy. Furthermore, large-scale dry etching simulations successfully formed etching holes over 2 nm deep.
The sample script for this computational case is available on matlantis-contrib: https://github.com/matlantis-pfcc/matlantis-contrib/blob/main/matlantis_contrib_examples/light_pfp_SiO2_HF_etching/SiO2-HF-etching.ipynb

Figure 1. Large-scale molecular dynamics simulation of SiO2 dry etching by HF gas.
Calculation models and methods
The calculations were performed in three distinct phases, as described below. Detailed computational conditions are provided in a separate “Computational Details”table.
Phase 1: LightPFP Model Construction
The LightPFP model was built through four stages:
1.Initial Structure Creation: We generated initial structures for SiO₂ crystals, SiO₂ surfaces, the etching gas HF, and various gas molecules produced during etching (e.g., SiF₄).
2.Initial Dataset Generation: Molecular dynamics (MD) calculations were used for sampling to create an initial dataset.
3.Active Learning for Data Collection: Active learning techniques efficiently gathered additional training data. This process involved 15 iterations, progressively increasing the complexity of etching conditions by varying slab sizes and the number of HF molecules.
4.LightPFP Model Training: The final LightPFP model was constructed by training it on the combined initial dataset, the active learning-collected dataset, and the pre-trained model “ALL_ELEMENTS_SMALL_NN_6”.
Phase 2: Reaction Pathway Analysis (LightPFP Model Performance Evaluation)
In Phase 2, we conducted NEB (Nudged Elastic Band) calculations to evaluate the LightPFP model’s performance. LightPFP results were then compared with those from PFP and prior research [1]. The initial state for the NEB calculations was defined as an SiO₂ cluster (Si₄O₁₀H₄) with an HF gas molecule. The final state represented fluorine adsorption on the Si surface, replacing oxygen, with an H₂O molecule detaching.
Phase 3: Large-scale Dry Etching Simulation
For Phase 3, we used the LightPFP model to simulate HF gas dry etching on a large-scale SiO2 slab model comprising approximately 72,000 atoms (10 nm × 10 nm × 10 nm).In this simulation, 1,000 HF gas molecules, each with a kinetic energy of 40 eV, were directed as a focused beam onto a 2 nm × 2 nm area of the slab surface.
Calculation Results and Discussion
Reaction Pathway Analysis of HF with SiO2 Cluster
Our reaction pathway analysis of HF with the SiO₂ cluster revealed that HF molecules adsorb onto the SiO2 surface, breaking Si-O bonds and forming Si-F bonds. LightPFP demonstrated accuracy comparable to PFP, with quantitatively consistent reaction barriers. PFP showed forward and reverse barriers of 1.029 eV and 1.561 eV, respectively, while LightPFP yielded 0.844 eV (forward) and 1.560 eV (reverse). These results also align with those from previous ReaxFF and DFT studies.
Significantly, the transition state structures were not deliberately included in the training data. This suggests that active learning effectively gathered these crucial structures directly from MD trajectories, thereby validating its efficacy in building the high-performance LightPFP model.
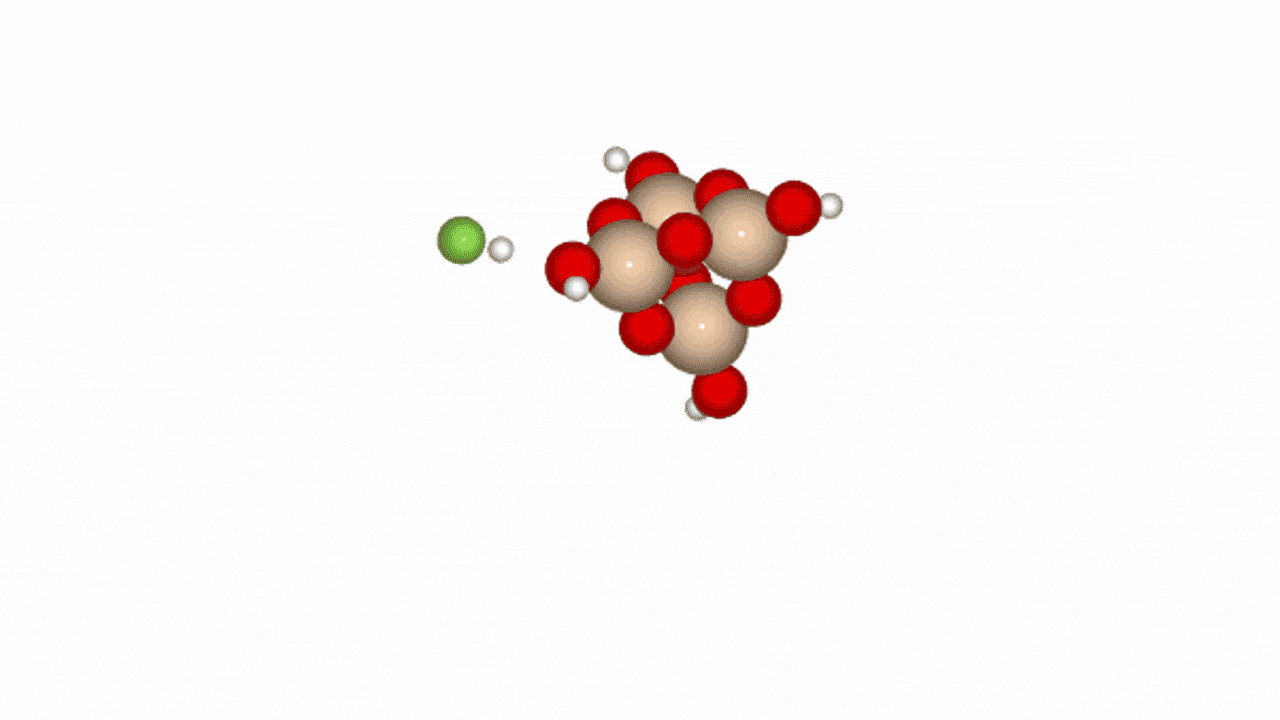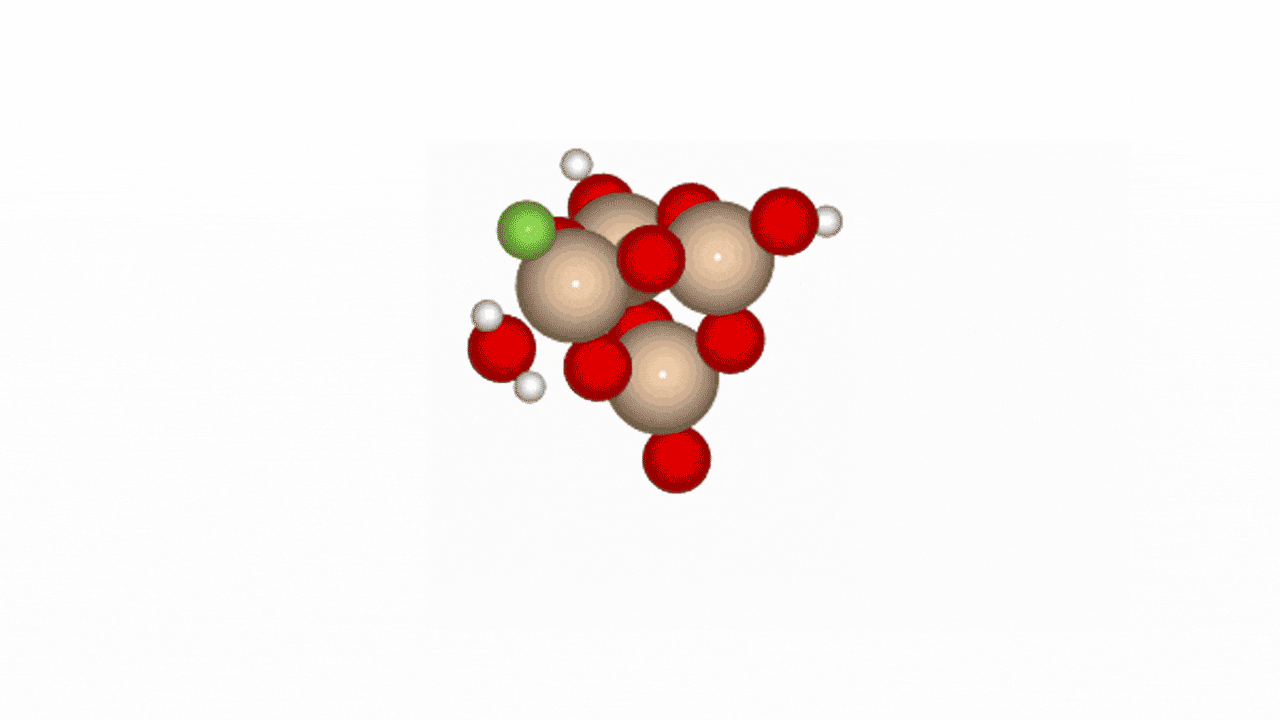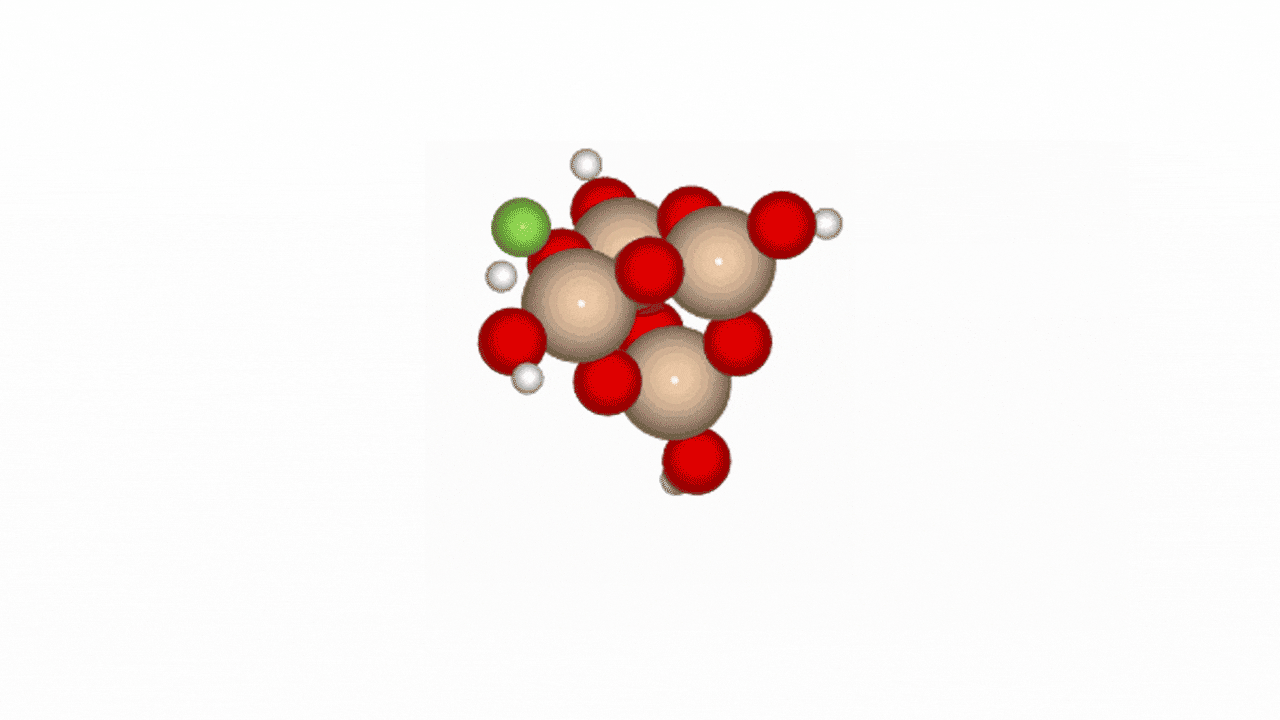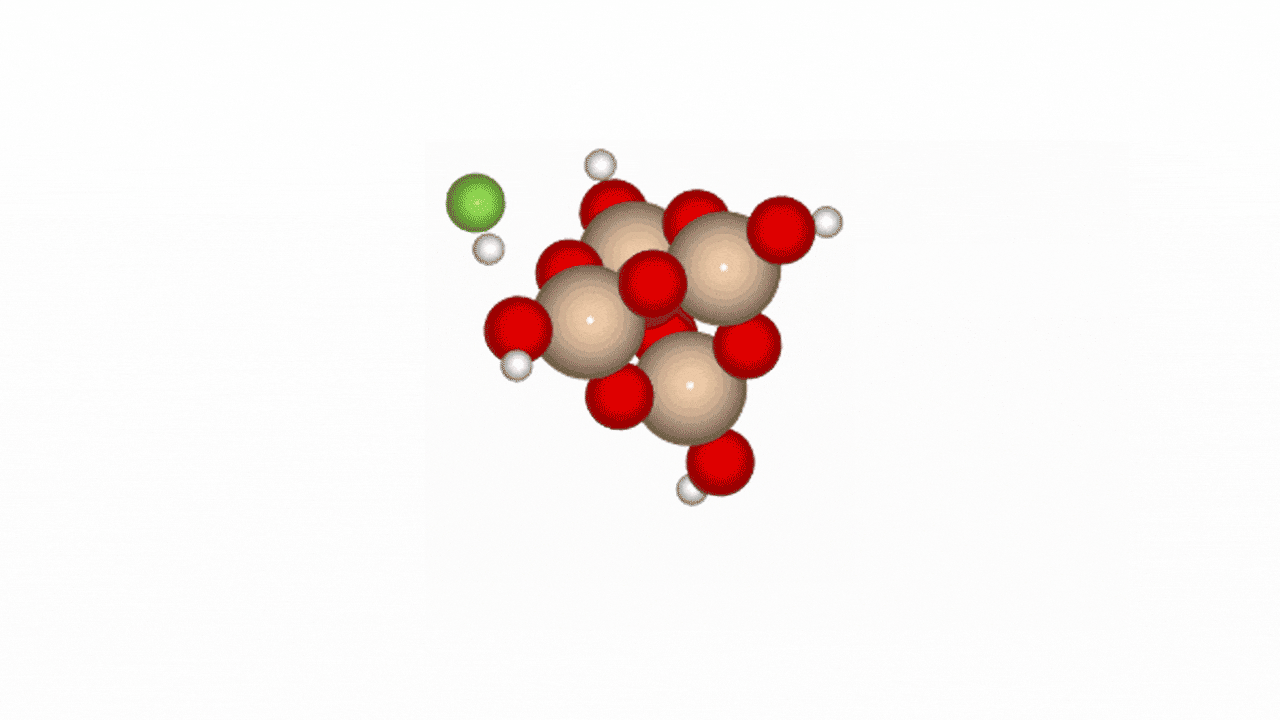
Figure 2. Reaction path of HF and SiO2 cluster
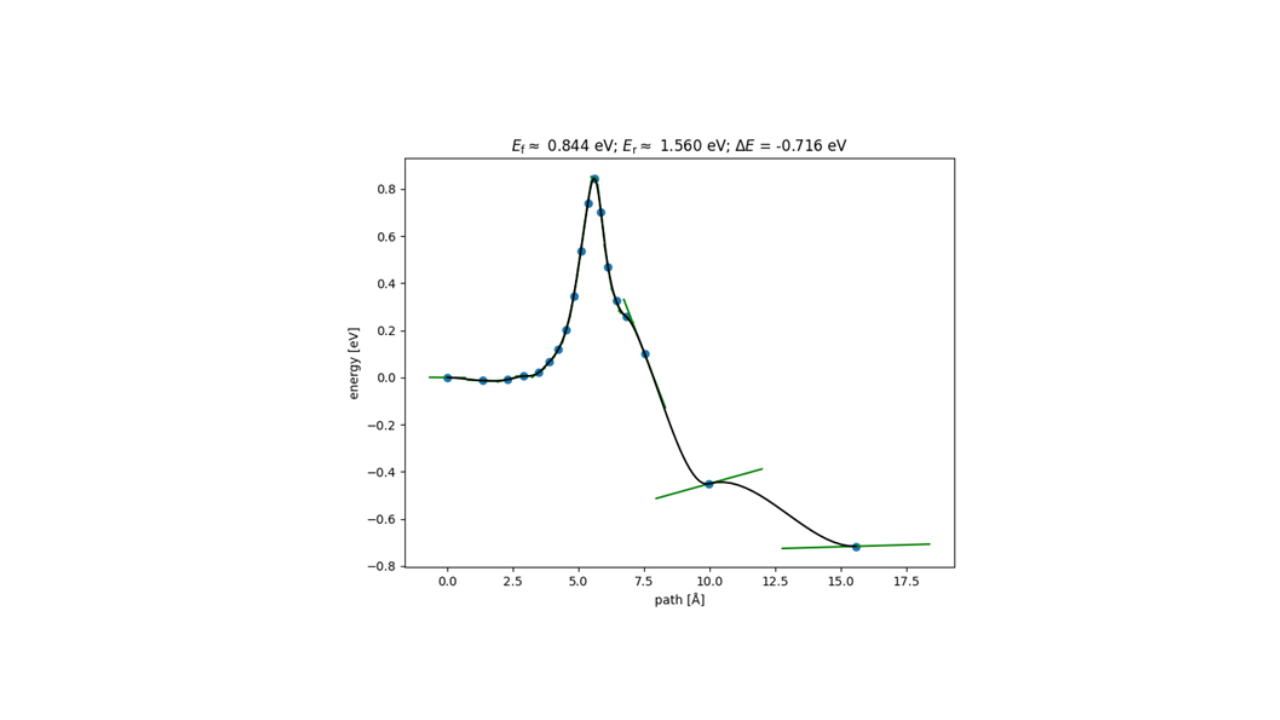
Figure 3. Energy Profile for HF Reaction Pathways on SiO₂ Cluster by LightPFP
Table 1. Enthalpy Change ΔH [kcal/mol] and Activation Energy Ea [kcal/mol]
| ΔH kcal/mol | Ea kcal/mol | |
|---|---|---|
| ReaxFF [1] | -19.78 | 42.64 |
| DFT [1] | -11.4 | 21.43 |
| PFP | -12.27 | 23.57 |
| LightPFP | -13.01 | 17.41 |
Large-Scale Dry Etching
In the large-scale etching simulation of the SiO₂ slab, we successfully created distinct etching holes by injecting 1,000 HF molecules into a localized area on the surface of a slab structure containing over 70,000 atoms. Over approximately 500 ps of MD simulation, the etching holes deepened to over 2 nm from the surface. The etching reaction led to the formation of Si-F bonds and the release of SiF₄ molecules. While conventional PFP or DFT struggle with simulations of this scale and duration, the superior speed of the LightPFP model allowed us to complete the simulation within a reasonable timeframe of about 45 hours.
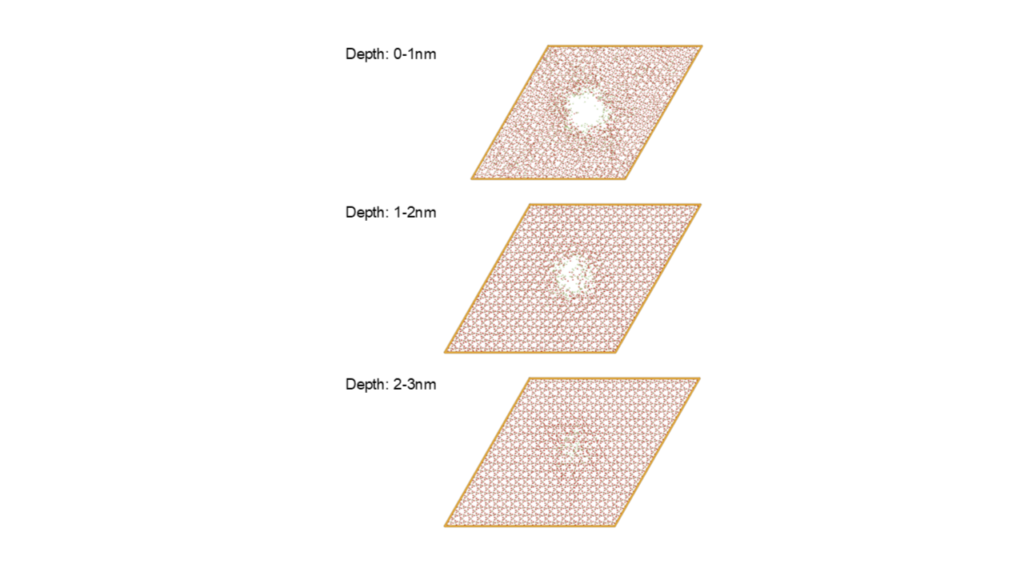
Figure 4. Structural Change of SiO₂ Slab After HF Gas Impact
Figure 5: Etching Hole Formation in SiO₂ Slab After HF Gas Impact
Future Prospects
The LightPFP model developed in this study holds significant potential for advancing dry etching technology in semiconductor manufacturing. It’s expected to provide insights for process control, including optimizing parameters like etching gas composition and incident energy, predicting the etching characteristics for various surface structures and materials, and enhancing selectivity with mask materials. Large-scale atomic simulations using LightPFP can visualize nanoscale processes that are challenging to observe experimentally, offering crucial information for process development.
Calculation Conditions
| Calculation Details |
|---|
| Common | |
|---|---|
| PFP Model Version | v7.0.0 |
| PFP Calculation Mode | crystal_u0_plus_d3 |
| LightPFP Model Development (2) Generation of initial dataset | |
|---|---|
| Sampling Temperature [K] | 1000, 3000, 5000 |
| Number of Steps | 5,000 steps at each temperature |
| Sampling Interval [step] | 100 steps |
| LightPFP Model Development (3) Active learning | |
|---|---|
| Number of Iterations | 15 Iterations |
| Script |
1.Insert one HF molecule with kinetic energy of 20–80 eV onto the SiO₂ surface. 2.Conduct 1,000 steps of NVE MD calculation with a timestep of 0.2 fs/step. 3.Conduct 500 steps of NVT MD calculation with a timestep of 1 fs/step. 4.Repeat steps 1–3 to inject multiple HF molecules |
| Iterations 0–4 | Insert 10 HF molecules into a small (2,2,1) size SiO₂ slab |
| Iterations 5–9 | Insert 20 HF molecules into a small (2,2,1) size SiO₂ slab |
| Iterations 10–14 | Insert 40 HF molecules into a large (4,4,1) size SiO₂ slab |
| Reaction Pathway Analysis | |
|---|---|
| Method | NEB/CI-NEB method |
| Spring Constant | k = 5.0 |
| Convergence Criteria | fmax = 0.07 |
| Number of Images | 19 |
| Large-Scale Etching Simulation | |
|---|---|
| System Size [atoms] | about 72,000 (SiO2 slab) |
| Number of HF Molecules | 1,000 molecules |
| Incident Energy [eV] | 40 |
| MD Simulation |
1.Geometry Relaxation: NVT 5 ps 2.Insert 1HF molecule: NVE 200 ps (0.5 fs/step) 3.Relaxation: NVT 300 ps (1 fs/step) ※ Repeat the above 2, and 3 for 1,000 cycles (total of 500 ps) |
| Total Simulation Time | about 45 hours |