A Statistical Understanding of Oxygen Vacancies in High-Entropy Perovskite Oxides
Case study provided by: Z-Energy Lab, Stanford University
Introduction
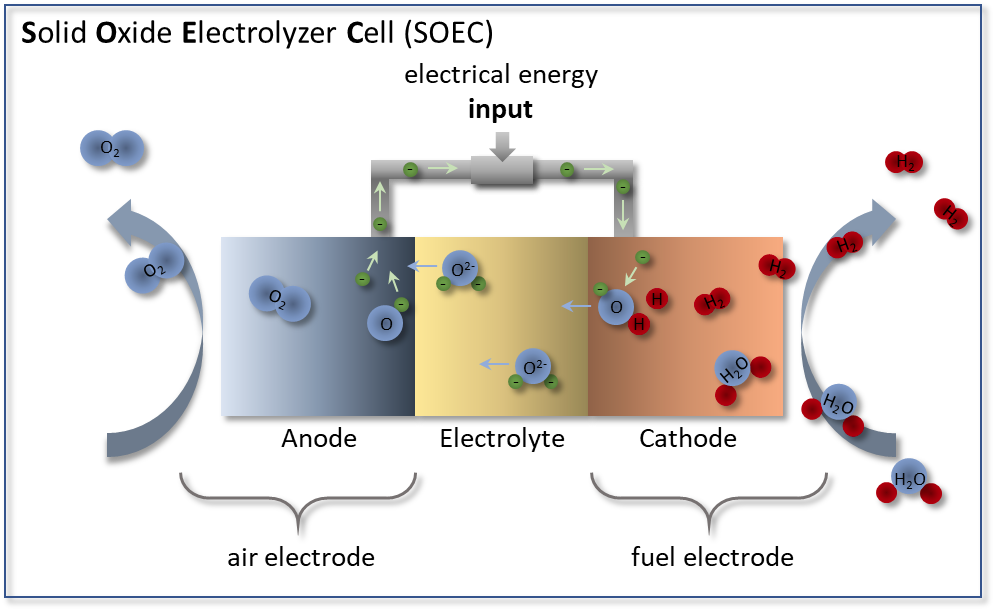
Solid oxide electrolyzers (SOEs) are a technology that generates renewable fuels, such as hydrogen, from electricity and waste heat with record high efficiency [1].
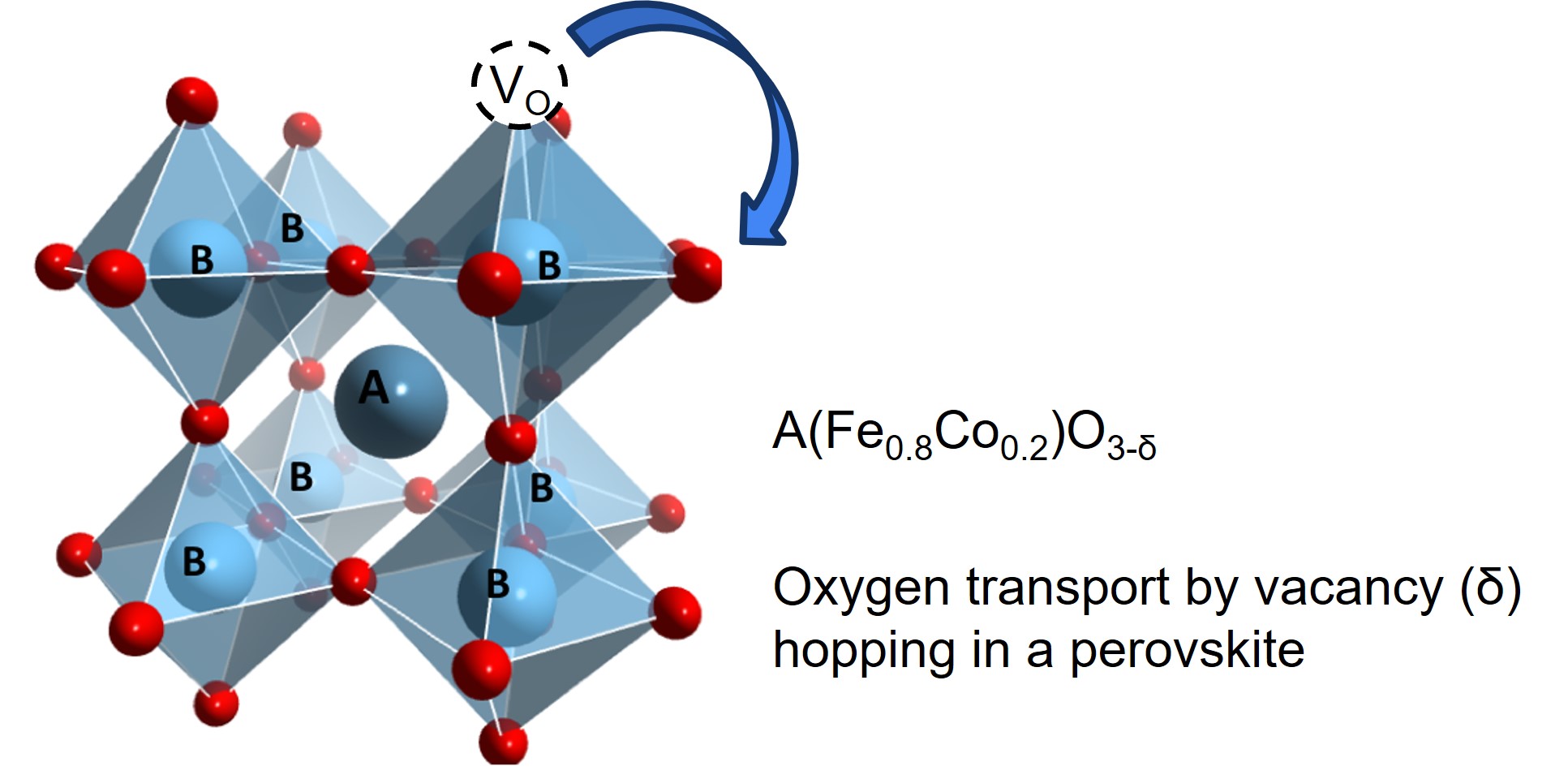
However, SOEs are limited by the performance of the anode material, which must effectively transport oxygen through oxygen vacancies. As such, it is critical to understand the forces driving oxygen vacancy concentration in perovskites (δ in ABO3-δ).
High-entropy perovskite oxides, which mix five or more elements to create a disordered crystal lattice, have shown promise as an electrode material, but their disordered composition makes them challenging to study with DFT.
Models & Calculation Methods (1)
Eight materials (two simpler low entropy-LE, six complex high entropy-HE) were studied using PFP and compared to experimental changes in oxygen vacancy concentration with increasing temperature.
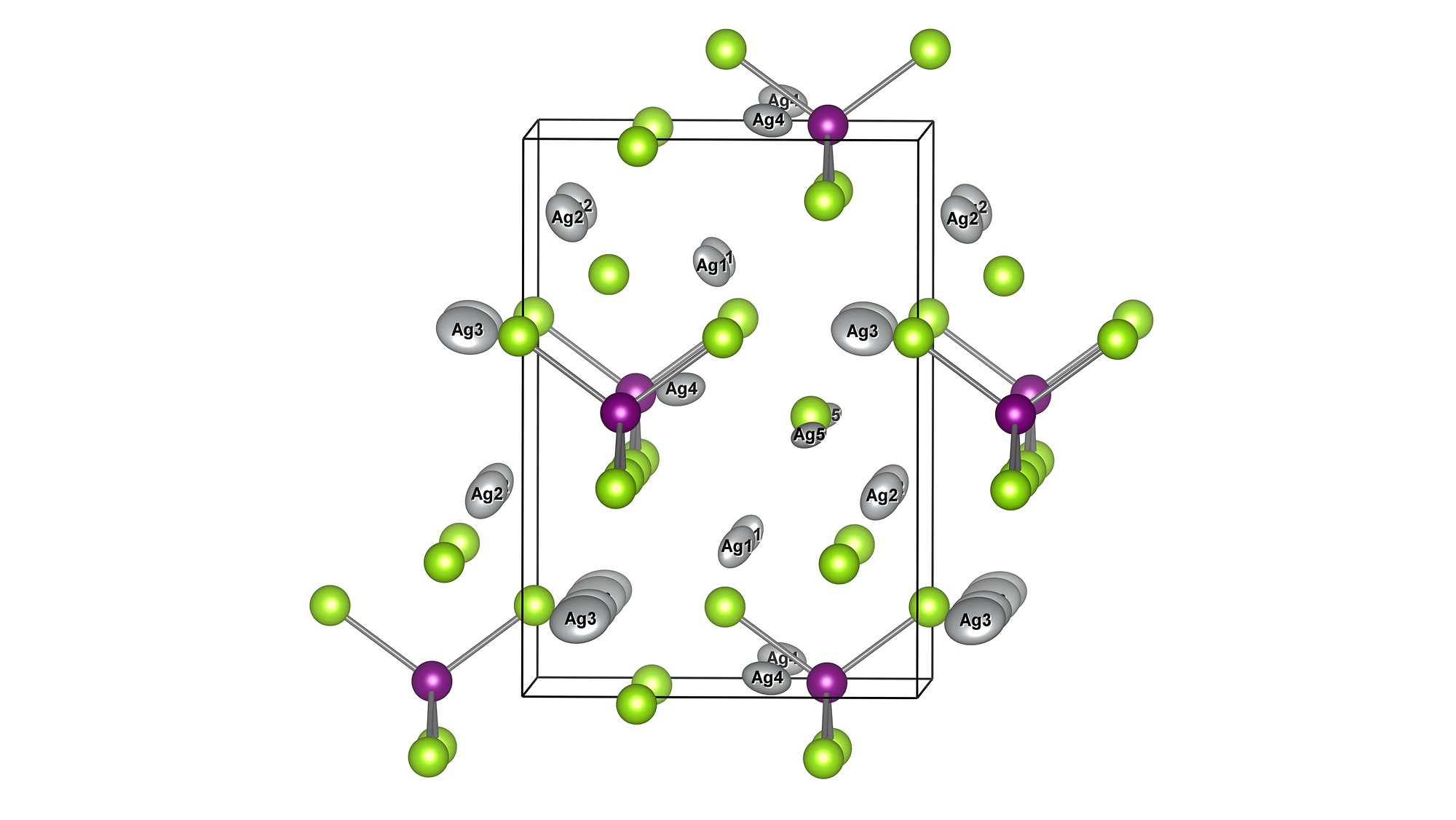
Large representative perovskite supercells were generated with random occupation of B-sites (Fe or Co) and A-sites (mixtures of La, Sr, Ca, Ba, Nd, Sm, Gd, Y) which vary by material. These large cells are necessary to represent the complex cation mixing interactions in high-entropy materials, but they are too large to scale with DFT simulations.
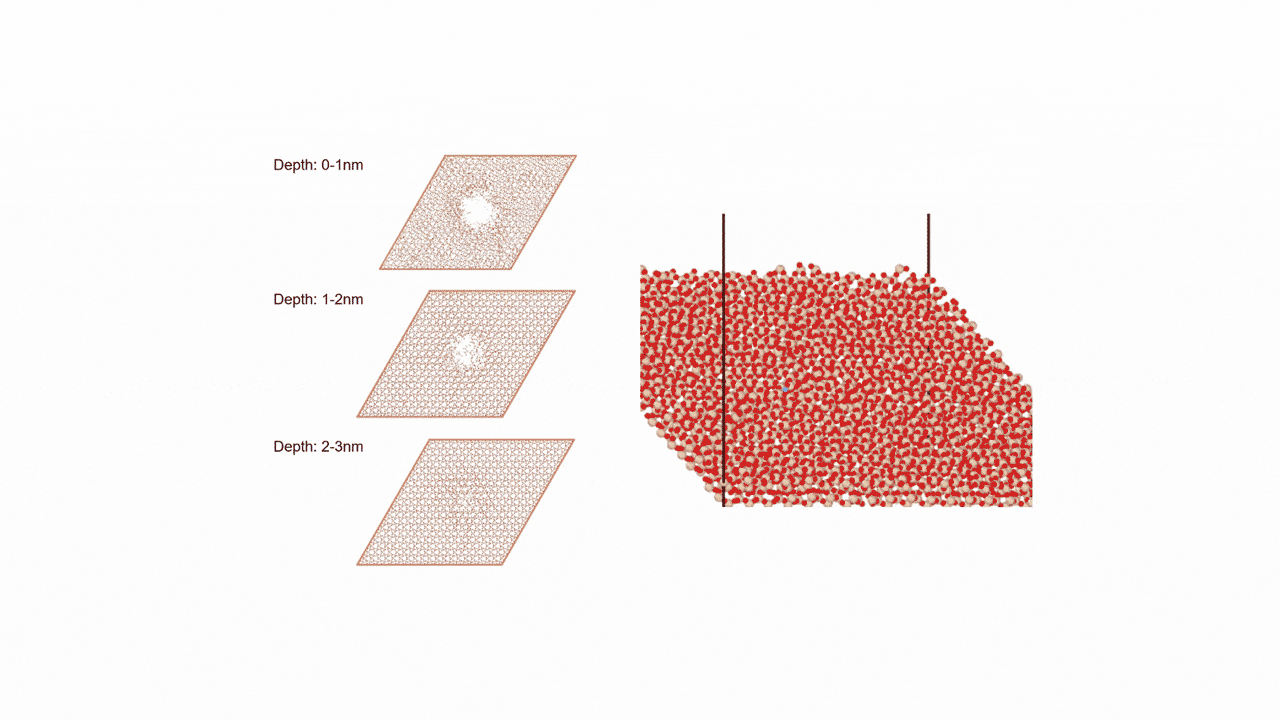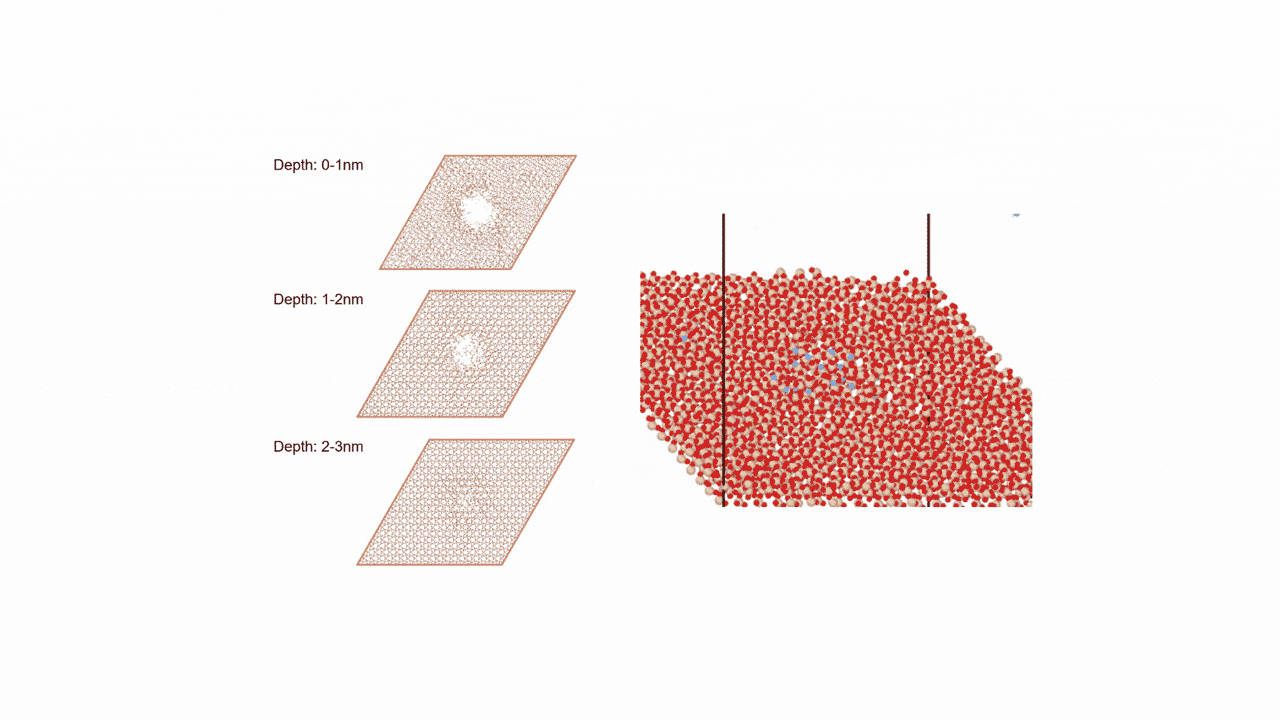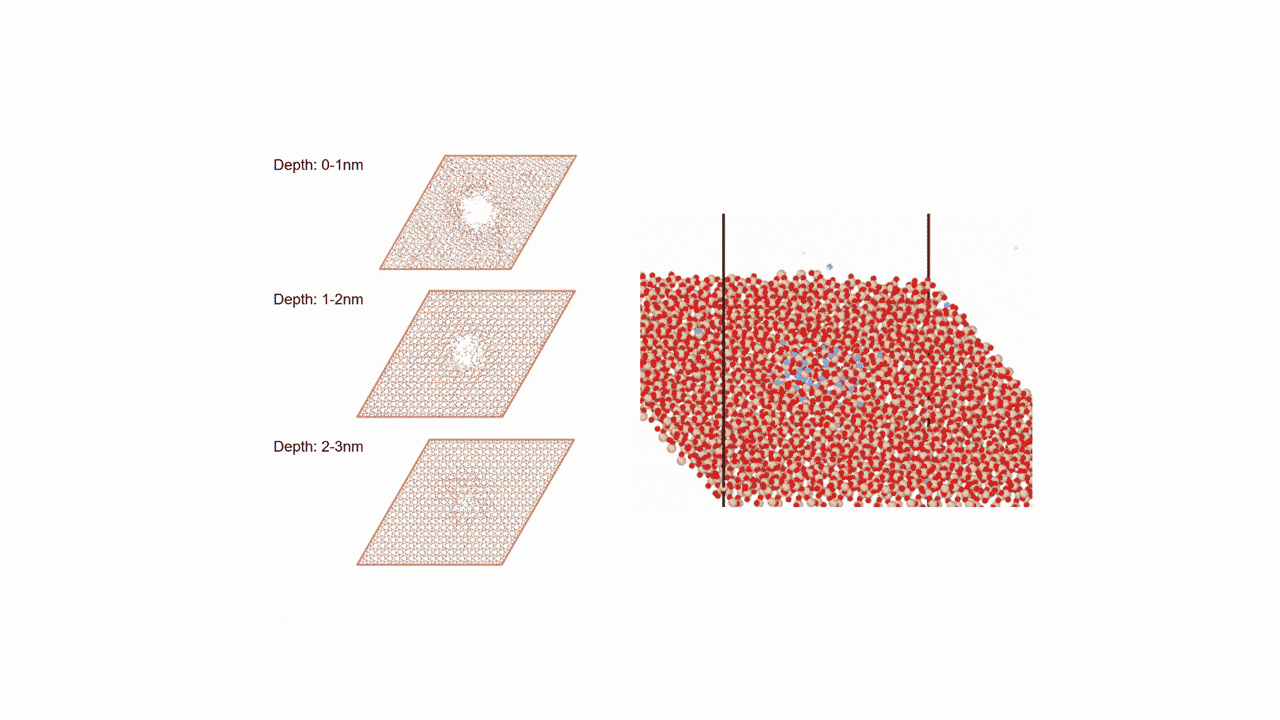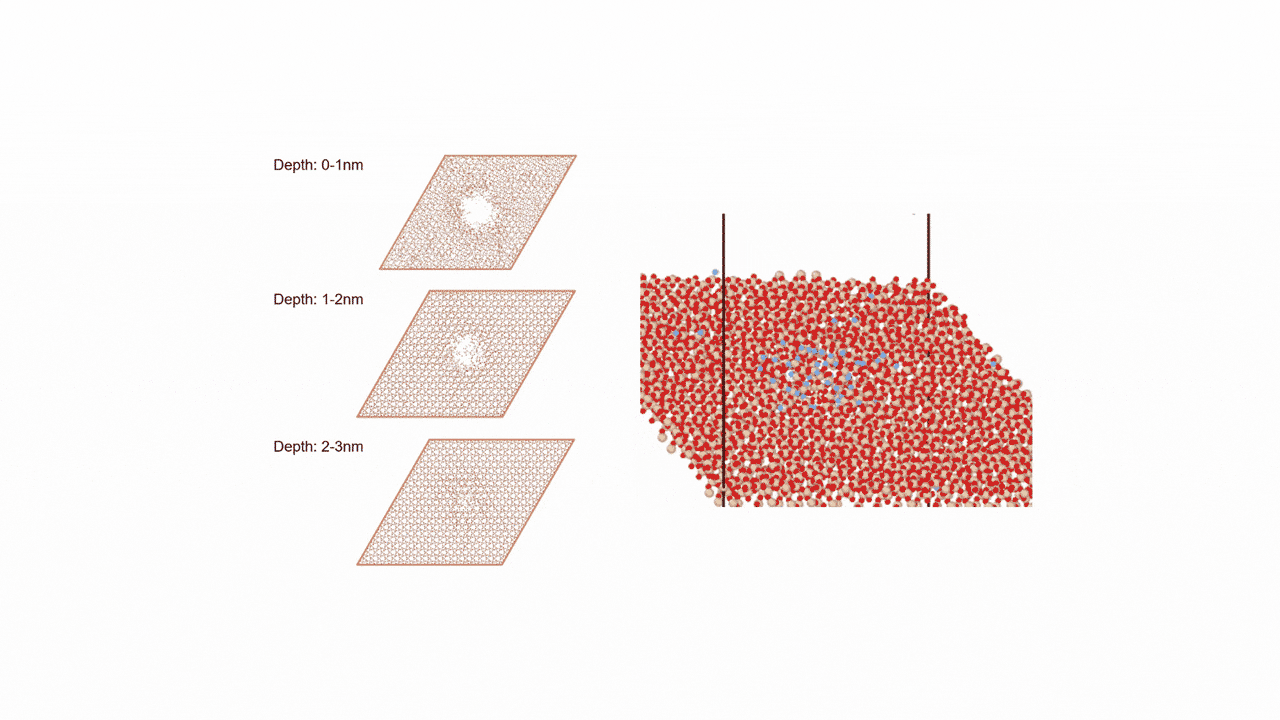
In each material, the oxygen vacancy energy is calculated for each of the N=768 oxygen sites by removing the oxygen and relaxing positions. The results is a distribution of vacancy energies for each material.
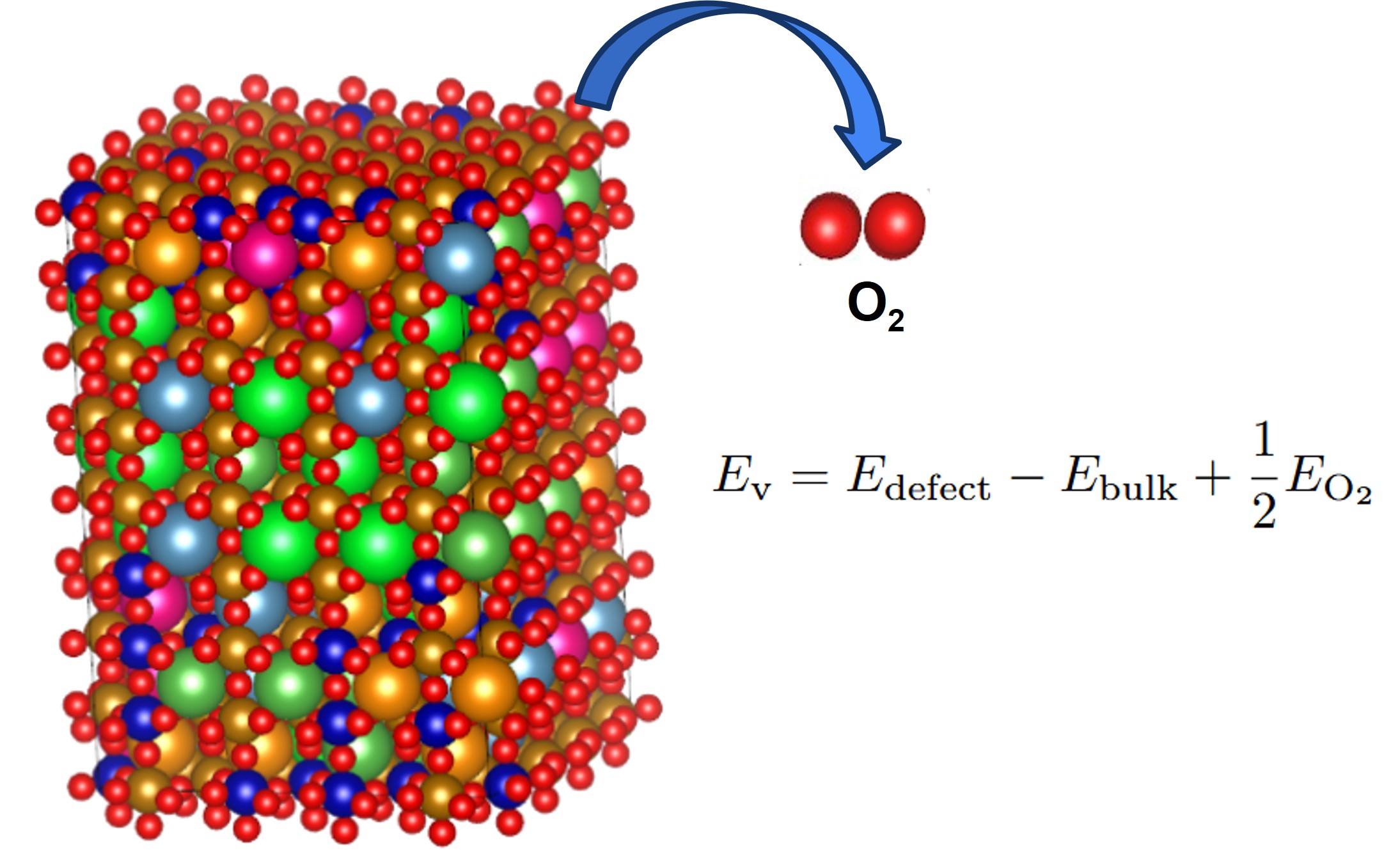
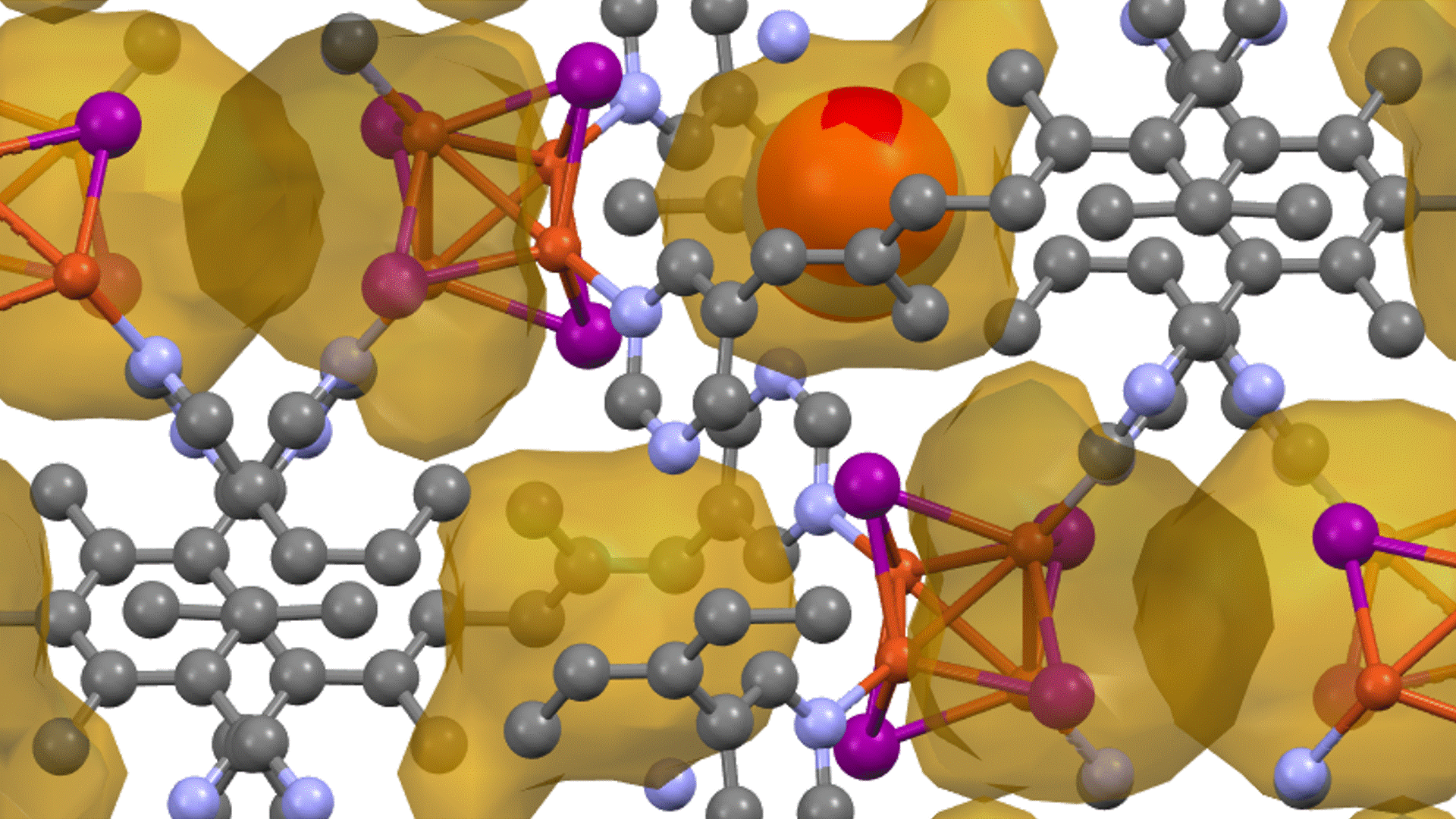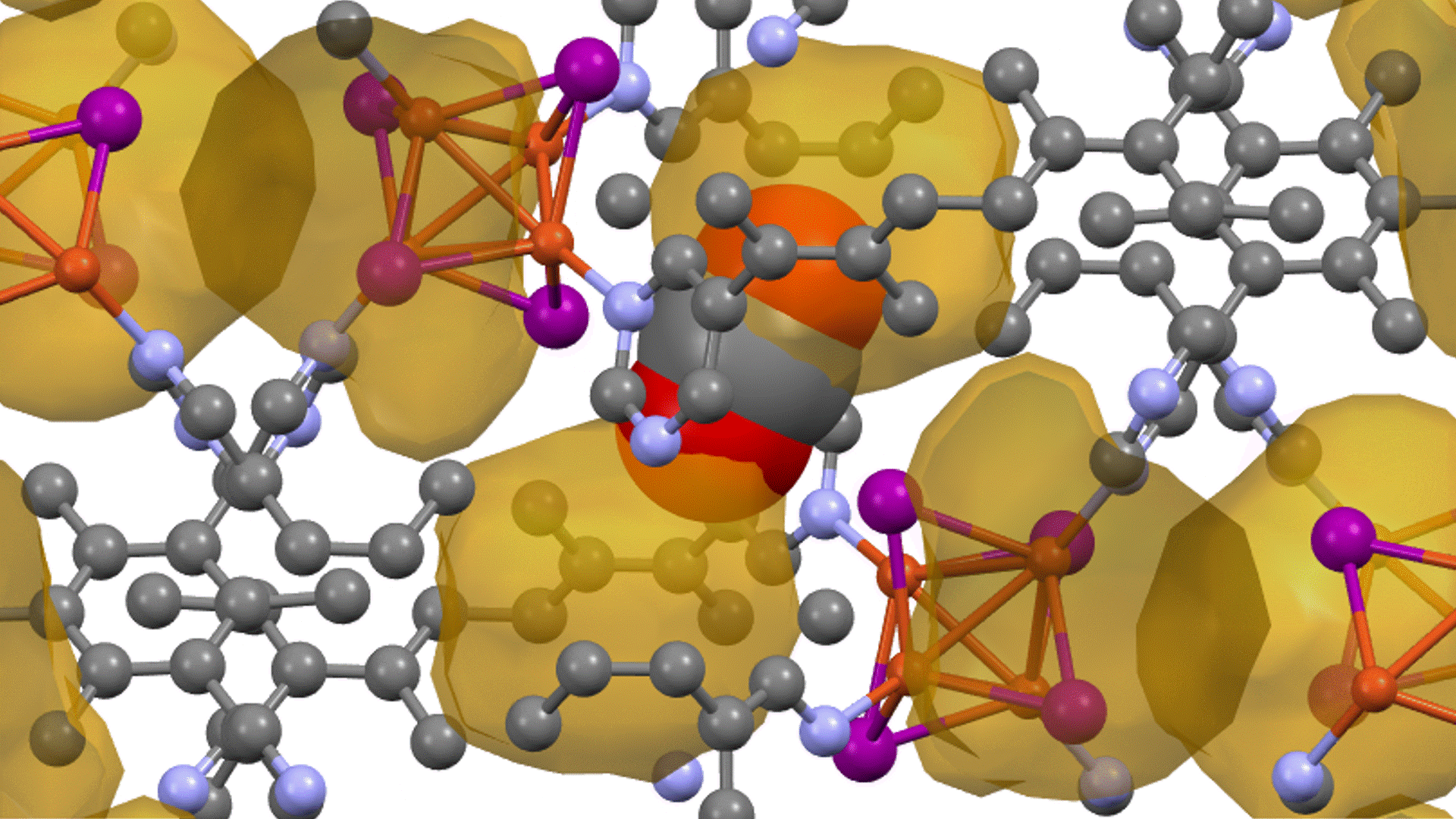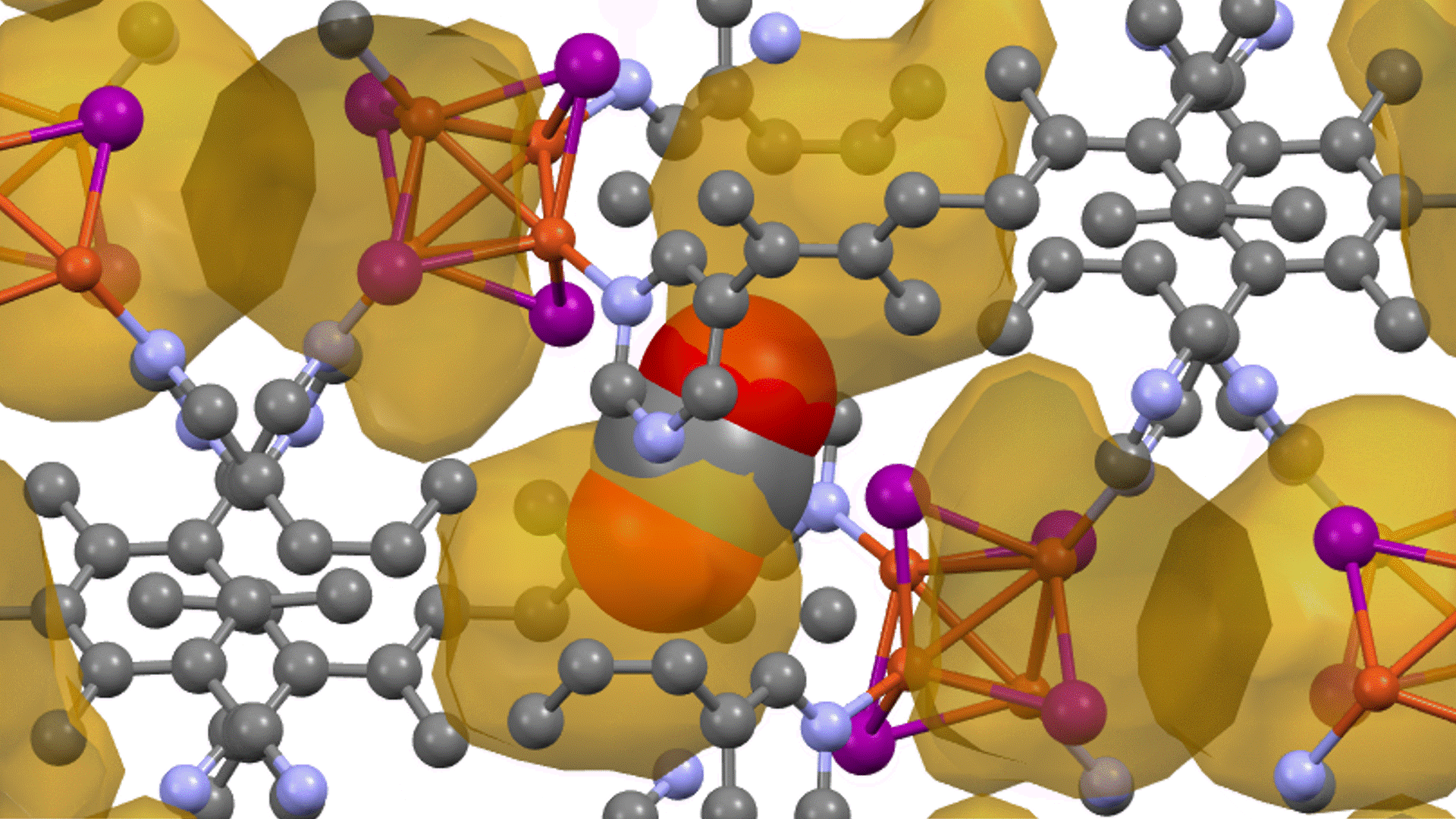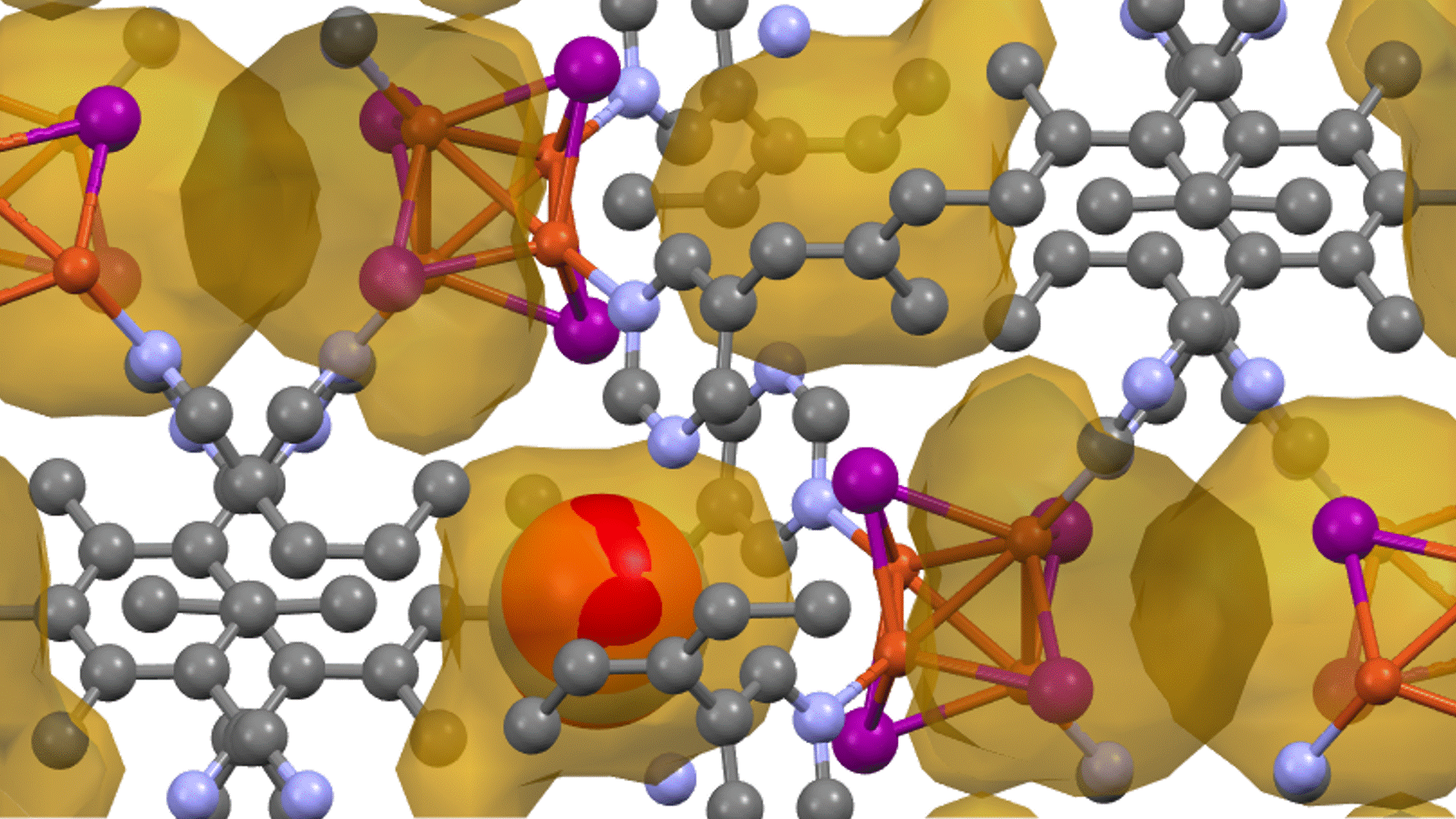
Supercell of high-entropy perovskite oxide with768 oxygen sites and random cation placement
Models & Calculation Methods (2)
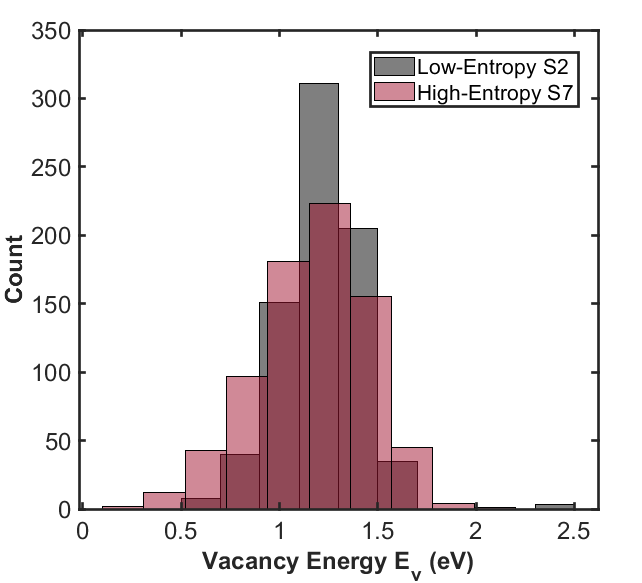
Traditionally, studies calculate a single value for the oxygen vacancy formation, but this study argues complex materials require a statistical averaging over all possible oxygen sites. The distribution of vacancy energy for two materials is shown including their average (![]() ). Increasing the variance in ionic radii among A-site cations (σA) appears to correlate with a broader distribution.
). Increasing the variance in ionic radii among A-site cations (σA) appears to correlate with a broader distribution.
Using methods from statistical thermodynamics [2], this study shows that the variance of the oxygen vacancy distribution can have substantial effects on the equilibrium vacancy concentration (δ) at a given temperature.
Results & Discussion
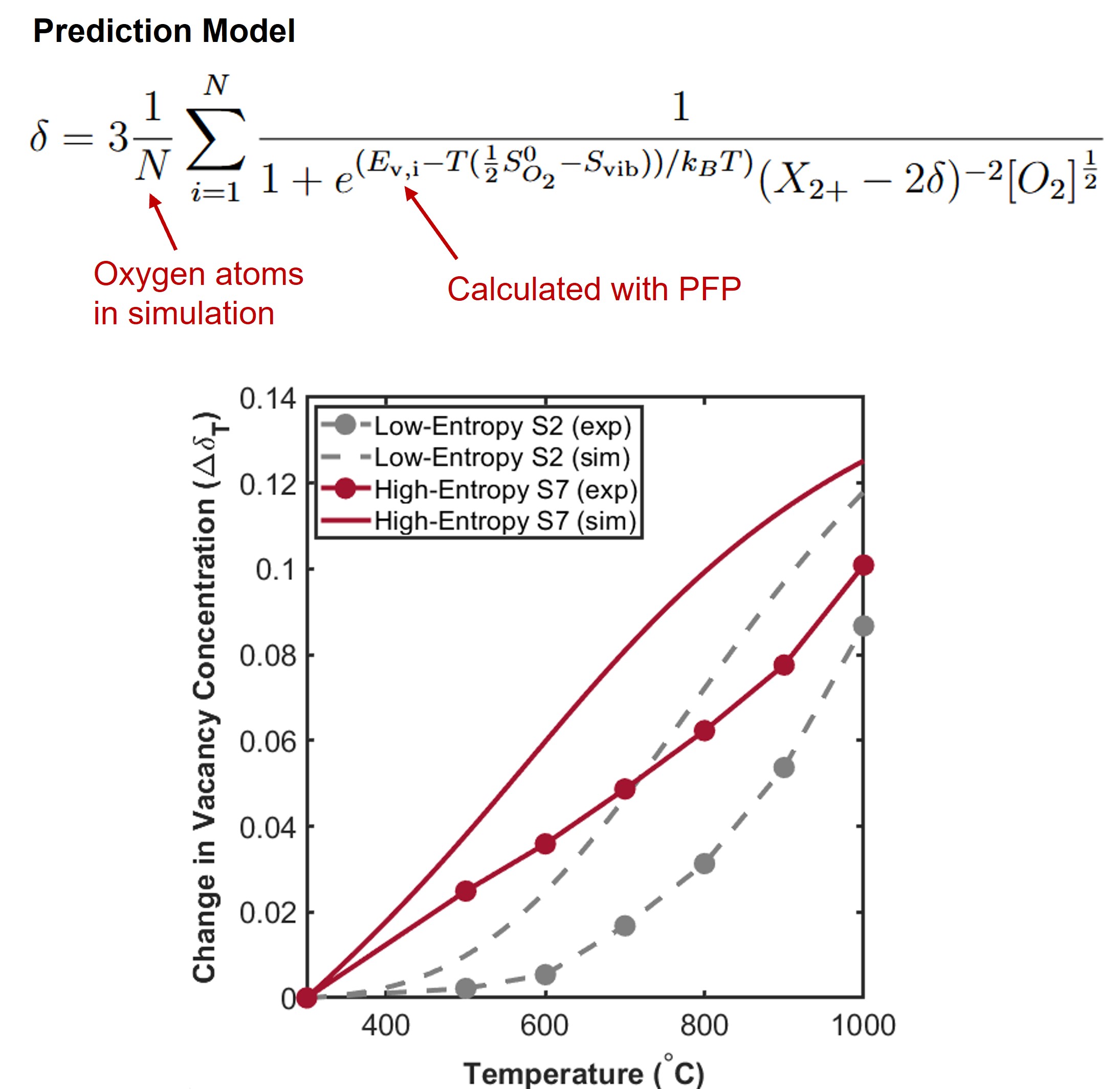
A statistical approach is proposed for estimating vacancy concentrations as a function of temperature and simulation sampled vacancy energies. The model is more accurate as the number of sampled oxygen (N) increases.
Using this model, simulations were able to predict relative differences in vacancy concentrations (Δ𝛿 𝑇 ) over a wide temperature range between a low-entropy and high-entropy oxide. This can guide the design of new electrode materials for SOEs.
This study leverages PFP’s flexibility to model oxygen, transition, alkali earth, and rare earth metals in one potential. In addition, PFP’s speed enabled the calculation of thousands oxygen vacancy energies making a new, more accurate statistical model possible.
Calculation Conditions
| Items | Details |
|---|---|
| Number of Atoms | 1280 atoms |
| Elements | O, La, Sr, Ca, Ba, Nd, Sm, Gd, Y, Fe, Co |
| Parameters | Model v7.0.0, CRYSTAL Volume and position relaxation fmax = 0.05 eV/A |
References
- H. Stormer. KIT Electron Microscopy, 2023
- M. T. Dove, Introduction to Lattice Dynamics, Cambridge Topics in Mineral Physics and Chemistry (Cambridge University Press).
Profile of The Case Study Provider
Z-Energy Lab, Stanford University

Publication date of this case: November 18, 2025