Around 7 p.m. on October 8, 2025, breaking news broke that "Professor Kitagawa of Kyoto University has won the Nobel Prize in Chemistry."
The Matlantis User Conference 2025 was held on the same day, and I was able to celebrate this good news with many Matlantis users and materials researchers who were in attendance. While I was still excited, I would like to explain the winning themes of "Porous Coordination Polymer (PCP)" and "Metal-Organic Frameworks (MOF)*."
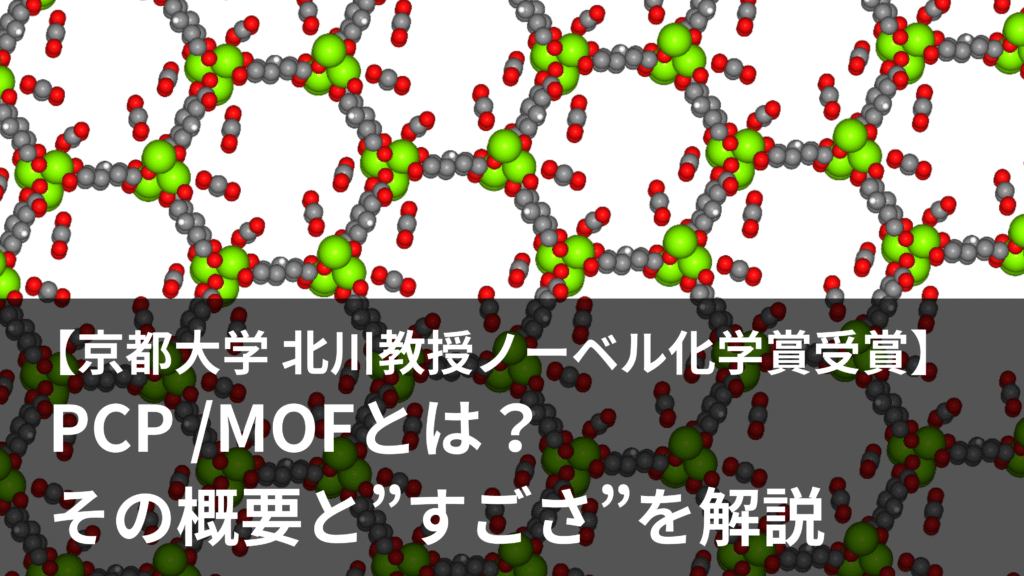
1. What is PCP/MOF?
Porous Coordination Polymers (PCP) or Metal-Organic Frameworks (MOF) are porous crystals composed of organic ligands and metal ions. Their greatest feature is that materials with a variety of properties can be easily synthesized by combining organic ligands and metal ions. They have attracted attention as they can be designed to perform gas adsorption, separation, and catalytic functions, and many researchers are currently conducting research into them.
I believe that the discovery of this series of materials and their subsequent developments led to this Nobel Prize being awarded.
2. Innovative features of PCP/MOF
Extremely large specific surface area > high adsorption performance
When PCP/MOF were first discovered, the thing that attracted the most attention was their large specific surface area (surface area per weight). Materials with a large specific surface area are considered to be advantageous for adsorbing molecules, and they are expected to be used as adsorbents. Activated carbon, which is used in refrigerator deodorizers, is also an excellent adsorbent, but some PCP/MOF boast a specific surface area several times larger than that, making them capable of adsorbing a correspondingly larger number of molecules.
Realization of uniform pores based on regular crystal structure > High molecular selectivity
Activated carbon has pores of various sizes, similar to the branches and leaves growing from the trunk of a broadleaf tree, and the state of these pores makes it possible to adsorb a variety of molecules. This is advantageous when you want to capture any odor-causing agent, such as in a deodorant. However, if you want to capture only specific molecules, it is necessary to make the pores uniform in size and characteristics. Creating uniform pores was itself a challenging task, but the emergence of PCP/MOF has opened up the prospect of materials capable of selective adsorption.
Combination of organic ligands and metal ions > High designability
Another important point is that the adsorption properties can be designed. In the case of general porous materials (activated carbon and zeolite), the general structure is fixed, and it is extremely difficult to significantly change the pore properties or to target molecules for adsorption (although there are methods such as surface modification).
However, PCP/MOF have a high degree of freedom, and by combining organic ligands and metal ions like blocks, it is possible to design pores with any diameter and adsorption properties.
3. Expected applications
Because the size and properties of the holes can be designed at the atomic level, it is expected that the material will be used to separate and recover only targeted materials, such as CO2, which causes global warming, valuable metal materials, and pollutants that are harmful to the environment and human body.
In addition, it is expected that adsorption and desorption will be more energy efficient than other materials. For example, if it is necessary to heat the material to 100 degrees Celsius to release molecules adsorbed at room temperature (25 degrees Celsius), the energy efficiency will be about three times higher than if it is heated to 50 degrees Celsius. This raises expectations for this material as a way to realize a sustainable world.
The practical application of PCP/MOF is already underway. Professor Kitagawa also serves as an advisor to Atomis, a Kyoto University-based venture company, which is promoting research and development aimed at their practical application. Matlantis is actually being used in the field of materials development.
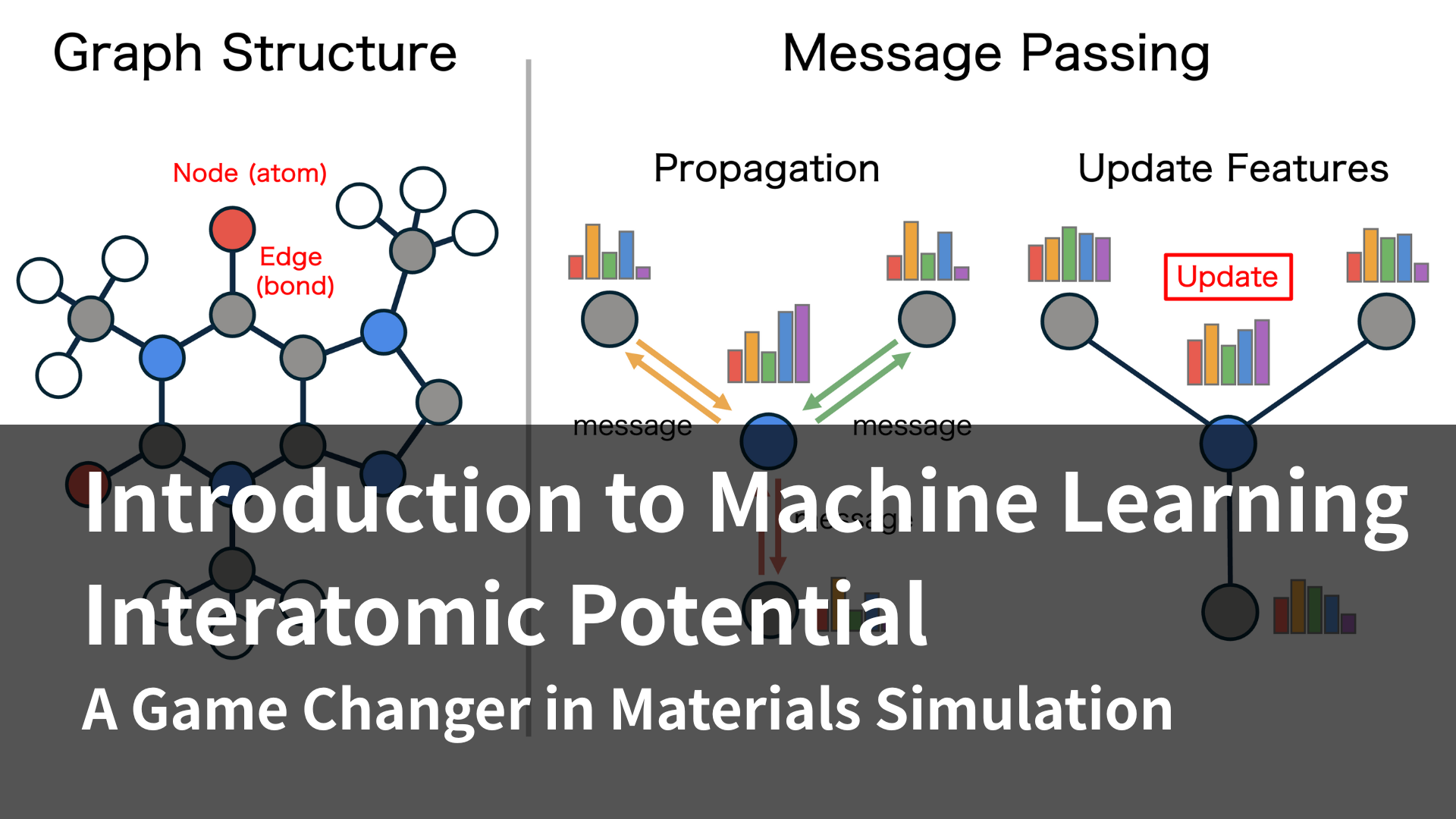
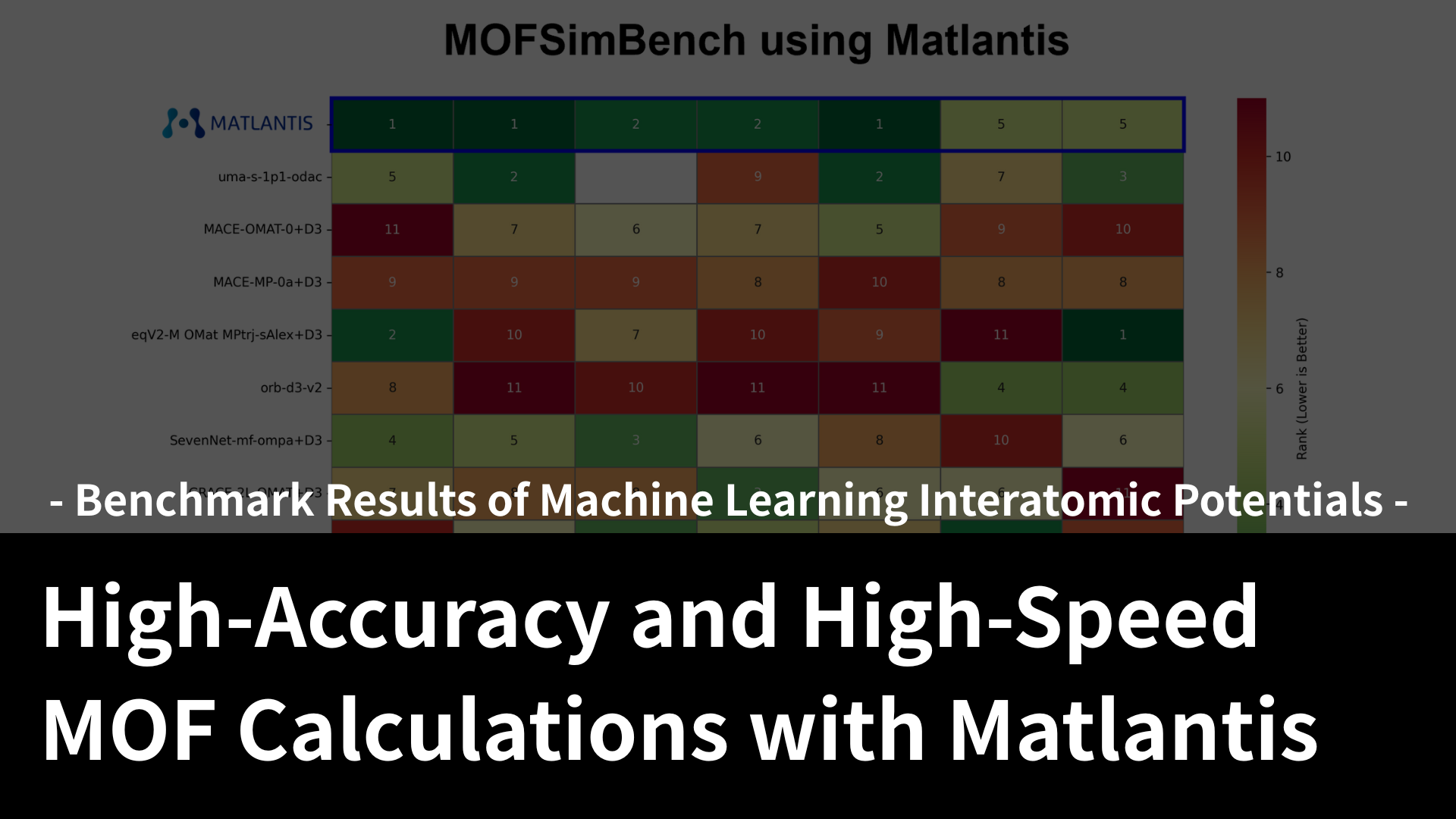
In order to develop highly functional materials, it is becoming essential to understand phenomena and design materials at the atomic level. (This is a blatant advertisement, but) our Matlantis software can perform large-scale calculations on a scale of tens of thousands of atoms at high speed with DFT accuracy, making it possible to perform simulations to understand phenomena such as the gas adsorption amount and adsorption selectivity of PCP/MOF.
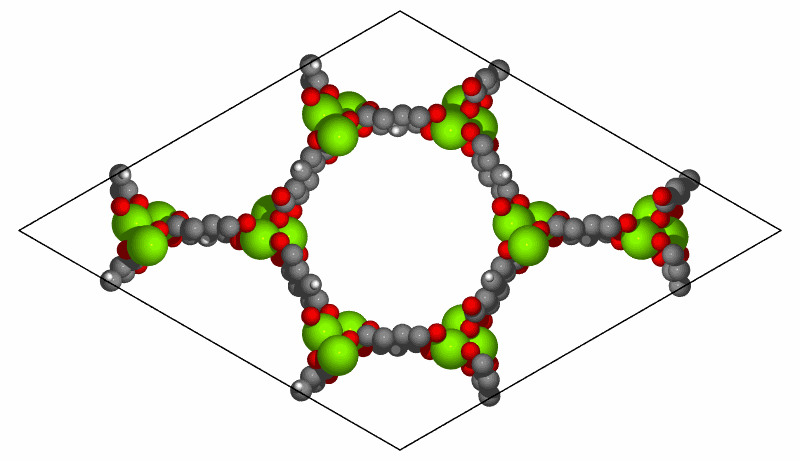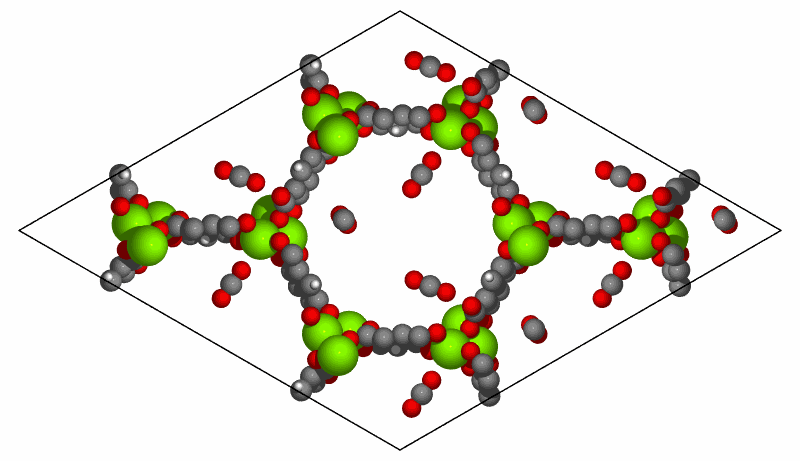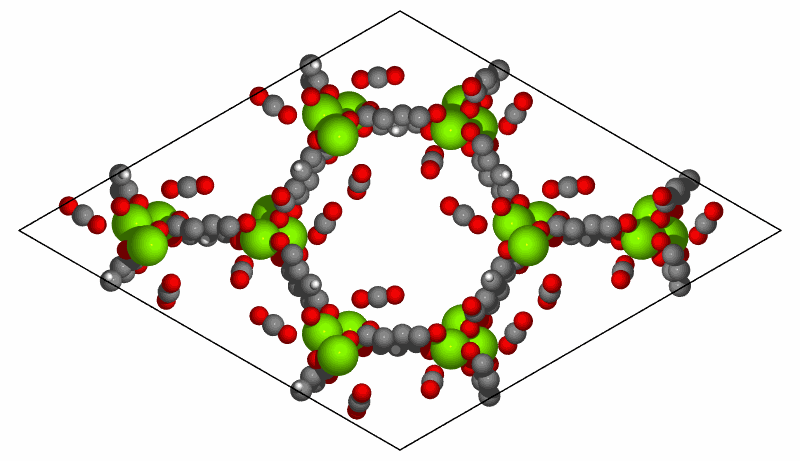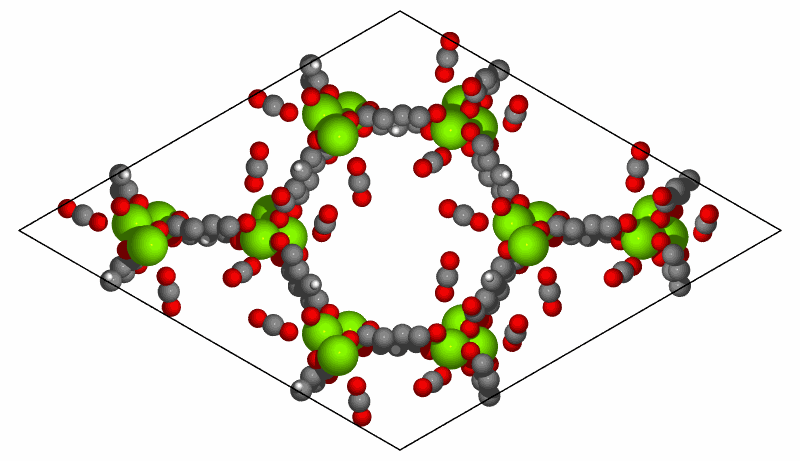
Simulation of CO2 adsorption on MOF-74(Mg) structure
Reference articles
Adsorption and diffusion behavior of H2O molecules in MOF-74Ni
[Calculation example published] Analysis of CO2 adsorption dynamics of MOF using the NEB method
5. Conclusion
"PCP/MOF have the potential to freely control adsorption/desorption and reactions at the atomic and molecular level, making them one of the important materials that will contribute to the realization of a sustainable world. This is an important scientific achievement, and I am truly pleased that you have received this award."